Joe DeMayo at all times knew his wholesome years might finish abruptly, certain to the lifespan of a transplanted kidney concerning the measurement of a small fist. However as the daddy of a toddler, he had hoped to have extra time.
When he was 33, his spouse had donated her kidney to him, a milestone that modified the course of DeMayo’s life. The relentless fatigue, nostril bleeds and itchy pores and skin introduced on by his personal poorly functioning kidneys vanished, and he felt ok to depart residence in Philadelphia for a brand new starting within the foothills of northern California.
Over lengthy afternoons, DeMayo would hike within the mountains along with his spouse and their black-and-white mutt, Fausto. When his son was born, he’d imagined himself teaching baseball video games, clad in Phillies gear.
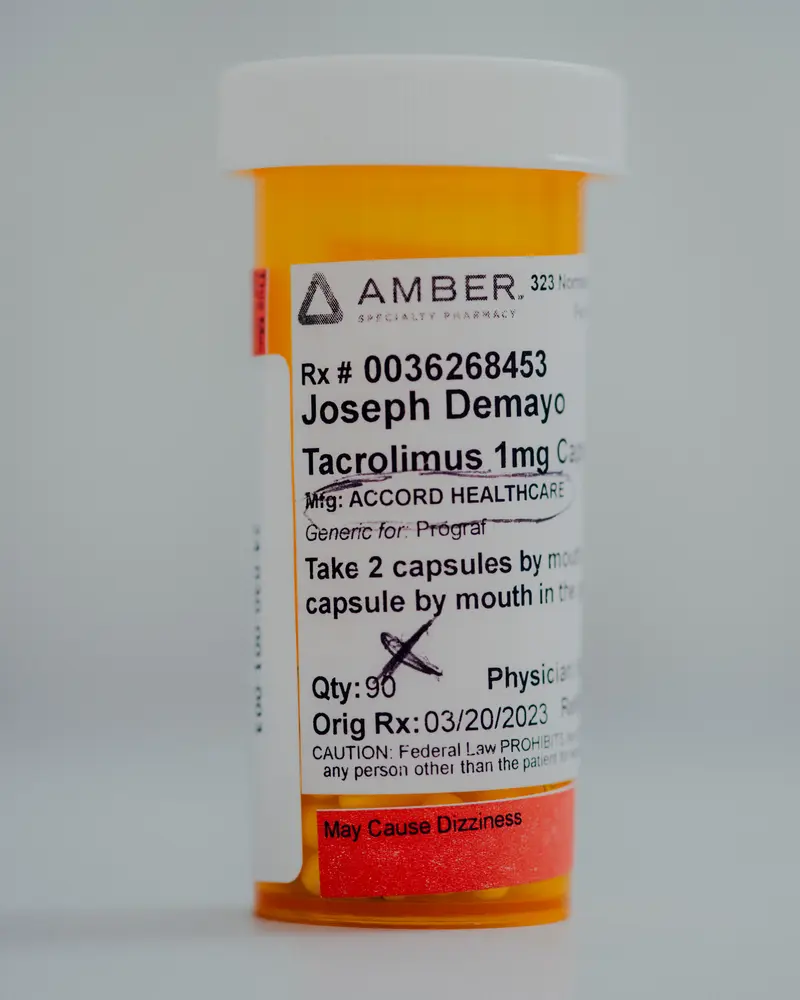
However his donated kidney began to fail in early 2023, a lot sooner than anticipated. The decline got here as a shock to DeMayo, who had been faithfully taking his drugs, together with tacrolimus, an important immunosuppression drug that helps stave off organ rejection.
Credit score:
Courtesy of Joe DeMayo
DeMayo didn’t know on the time that the capsules he swallowed twice a day exactly 12 hours aside might have left him weak — or that one of the crucial formidable drug regulators on the planet could have failed to guard him.
As he grew weaker, his kidney unable to cleanse his physique of extra fluid and waste, investigators from the Meals and Drug Administration headed to western India to examine the manufacturing unit that manufactured DeMayo’s tacrolimus and different generic medicine for American shoppers.
It was not less than the eighth time since 2015 that the FDA had been there, and every of these visits had uncovered issues in the way in which the medicine have been made, authorities data present.
In the course of the inspection within the spring of 2023, investigators found the Intas Prescribed drugs manufacturing unit had, amongst different issues, manipulated drug-testing records to cowl up the presence of particulate matter — which might embrace glass, fiber or different contaminants — within the firm’s medicine.
Unaware of the inspection, DeMayo continued taking his tacrolimus capsules. He fought exhaustion and struggled to carry onto his job behind a deli counter.
“Daddy wants a brand new kidney,” he recalled telling his 5-year-old son on the time.
Credit score:
George Etheredge, particular to ProPublica
That November, the FDA barred the Intas manufacturing unit from exporting medicine to america. However underneath a long-standing practice uncovered by ProPublica, the company excluded sure drugs from the factory-wide ban, together with tacrolimus, permitting the medicine to proceed flowing to the U.S.
In an announcement to ProPublica, Intas, whose U.S. subsidiary is Accord Healthcare, mentioned that the corporate couldn’t touch upon the instances of particular person sufferers however that its tacrolimus is secure and efficient. The corporate mentioned it instantly responded to the FDA’s inspection findings, launching a program centered on high quality and investing hundreds of thousands of {dollars} in upgrades and new hires. Intas additionally mentioned that some exempted medicine have been by no means shipped to america however wouldn’t present particulars.
“Intas is nicely on its approach in direction of full remediation of all manufacturing websites,” the corporate mentioned.
ProPublica’s investigation discovered the FDA has allowed greater than 150 medicine or their substances from banned factories into the nation over the previous dozen years, ostensibly to stop drug shortages.
The company didn’t routinely check the medicine or actively search for indicators of sudden or unexplained reactions amongst sufferers. And the exemptions have been largely saved hidden from Congress and the general public, together with sufferers like DeMayo, who counted on his medicine to maintain him alive.
DeMayo crammed one other prescription for tacrolimus solely days earlier than the FDA exempted it from the Intas import ban and continued taking the capsules till simply earlier than his second transplant surgical procedure at Temple College Hospital in January 2024.
“I’m attempting to do the proper factor, take all my drugs,” mentioned DeMayo, 45, who took Intas tacrolimus for 2 years. “If I’m doing all that, shouldn’t any individual be doing their due diligence?”
In an announcement, the FDA mentioned drugmakers that obtain a cross from import bans are required to conduct extra security and high quality testing and rent third-party specialists to evaluate the outcomes earlier than delivery medicine to america. Present and former FDA officers mentioned these measures are defective. Lots of the firms have been cited earlier than for testing protocols that have been ineffective or liable to fraud.
DeMayo, now recovered from his second transplant surgical procedure, gave ProPublica two bottles of his unused Intas tacrolimus capsules. ProPublica had them examined at Valisure, an independent, accredited lab in Connecticut.
Of their first check, the scientists at Valisure discovered that a few of DeMayo’s drugs contained an enough quantity of the important thing ingredient however others contained a decrease quantity than the minimal stage set by U.S. regulation. Pharmacists, medical doctors and different specialists mentioned underdosing can depart sufferers weak to organ rejection.
Valisure didn’t discover any substantive contamination in DeMayo’s medicine.
However the scientists discovered one other potential drawback. The capsules dissolved rapidly — as much as thrice sooner than the identify model. Fast dissolution can introduce an excessive amount of of the drug too rapidly, specialists mentioned, doubtlessly inflicting tremors, complications and kidney failure.
ProPublica didn’t check tacrolimus made by another producer. In its assertion, Intas mentioned that the findings are “unrelated to the [FDA’s] inspections” and that the FDA had decided the drug was equal to the brand-name model when it was first authorised for the U.S. market.
Valisure beforehand examined Intas’ tacrolimus for the Division of Protection, which is conducting safety and quality testing on greater than three dozen medicine generally utilized by U.S. service members and their households. These exams, too, confirmed the capsules dissolved too rapidly.
“That is an alarming sign of different high quality points that may be affecting affected person care,” mentioned retired Military Col. Vic Suarez, who helped launch the Protection Division effort and is aiding on the challenge.
The FDA carried out its personal studies of Intas’ tacrolimus lately and reported the same consequence on its web site. The company famous there was no obvious threat of organ rejection however mentioned the Intas generic might create toxins within the physique, which might trigger kidney injury. The FDA mentioned the capsules could not present the identical therapeutic impact because the brand-name model.
The findings were made public in September 2023. Weeks later, the company went on to excuse the drug from the Intas import ban, permitting the corporate to proceed delivery tacrolimus to america.
Janet Woodcock, who for years led the FDA’s Heart for Drug Analysis and Analysis, mentioned in an interview that the outcomes of the testing are regarding and that the company ought to rapidly “attempt to kind them out.”
“This clearly was a high quality drawback,” she mentioned.
Woodcock didn’t say why the FDA exempted the drug from the import ban imposed on the Intas manufacturing unit. Although Woodcock authorised exemptions for years, she had left the middle and was serving because the FDA’s principal deputy commissioner when the exemptions for tacrolimus and different Intas medicine have been made.
DeMayo mentioned he’ll by no means know whether or not the medicine contributed to the lack of his donated kidney. Organ rejection, which might occur rapidly or over years, is among the many most common causes of kidney failure in transplant sufferers, however kidneys can fail for different causes, too, mentioned Joseph Vassalotti, chief medical officer on the Nationwide Kidney Basis.
In DeMayo’s case, he was hospitalized with a abdomen virus and dehydration the identical yr his kidney operate began to say no. Nonetheless, he questions the drug that was supposed to guard him and worries that different transplant sufferers who’ve taken Intas tacrolimus may very well be in danger.
One and a half years after the FDA banned the manufacturing unit from delivery medicine to america, tacrolimus continues to be excluded. A customer support agent for the corporate mentioned Intas just lately stopped distributing the drug, however the firm didn’t reply to a request for remark.
“The individuals who oversee the drugs are failing and the people who find themselves making the drugs are failing,” DeMayo mentioned. “How did it get so unhealthy?”
Credit score:
First and third images: Hannah Yoon for ProPublica. Second picture: George Etheredge, particular to ProPublica.
Lucas Waldron contributed graphics and improvement.









